| 101 | Wetted length (given liquid transport in porous media, sorptivity, porosity ) | -494s.png) |
| 102 | Liquid-air surface tension (Capillary action) | -495s.png) |
| 103 | Density of liquid (given liquid-air surface tension,capillary action) | -496s.png) |
| 104 | Radius of tube (given liquid-air surface tension,density,height of liquid column, Capillary action) | -497s.png) |
| 105 | Volume of the pores (Measuring porosity by gas expansion method) | -498s.png) |
| 106 | Porosity (Measuring by gas expansion method) | -499s.png) |
| 107 | Specific heat at constant pressure (Mayer\'s Relation) | -500s.png) |
| 108 | Specific heat at constant volume (Mayer's Relation) | -501s.png) |
| 109 | Heat capacity at constant pressure for ideal gas (Given heat capacity at constant volume) | -502s.png) |
| 110 | Heat capacity at constant volume for ideal gas (given heat capacity at constant pressure) | -503s.png) |
| 111 | Dimensionless heat capacity (given the amount of substance) | -504s.png) |
| 112 | Dimensionless heat capacity (Given the number of molecules in the body) | -505s.png) |
| 113 | Molar heat capacity of an ideal gas (Given degrees of freedom) | -506s.png) |
| 114 | Enthalpy of system (Given Gibbs free energy,internal energy and Helmholtz free energy) | -508s.png) |
| 115 | Internal energy of the system (Given enthalpy,pressure and volume) | -509s.png) |
| 116 | Internal energy of the system (Given Helmholtz free energy,temperature and entropy) | -510s.png) |
| 117 | Internal energy of system (Given Helmholtz free energy,Gibbs free energy and enthalpy) | -511s.png) |
| 118 | Helmholtz free energy (Given internal energy, temperature and entropy) | -512s.png) |
| 119 | Helmholtz energy (Given internal energy, enthalpy and Gibbs free anergy) | -514s.png) |
| 120 | Gibbs energy (Given internal energy, enthalpy and Helmholtz free anergy) | -515s.png) |
| 121 | Entropy of the system (Given Gibbs free energy and enthalpy) | -517s.png) |
| 122 | Entropy of the system (Given Helmholtz free energy and internal energy) | -518s.png) |
| 123 | Change in entropy with constant heat and volume | 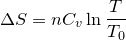 |
| 124 | Change in entropy with constant heat and pressure | 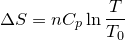 |
| 125 | Change in entropy with constant heat and temperature | 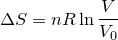 |