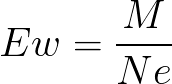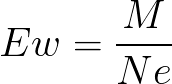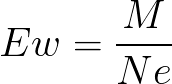Equivalent weight of oxidizing agents
Formula
Ew
equivalent weight of oxidizing agents
Ne
Number of electrons gained by one molecule
Formula description
In acid-base chemistry, normality is used to express the concentration of protons (H+) or hydroxide ions (OH-) in a solution. Each solute can produce one or more equivalents of reactive species when dissolved.